This website is not associated with any club, group or association. It is a private website providing information for the home wine maker.
El Dorado Home Wine Making Website - Home Wine Making Information and Wine Recipes

Wine Wallpaper
Donate and Help Out
If you have found this website to be helpful, maybe you might want to donate a couple dollars to show your appreciation.
Wine Making Information
Tasting Descriptors
| Brettanomyces | Unfavorable Malo-Lactic Bacteria |
|---|---|
| damp earth | saukruet |
| dank cellar | dill pickle |
| wet dog | spoiled milk |
| horse blanket | cheesiness |
| sweating horse | tanky |
| gym socks | vinous(favors mask varietal characters so all wines taste the same) |
| gyn shoes | rubbery |
| raw peanuts | gassy (in the bottle) |
| Acetic Acid Bacteria | Mycoderma - Film Yeast |
|---|---|
| vinegar | flat |
| glacial acetic acid | dull |
QUICK WINE TASTING COURSE
What's the point in tasting wine? This is a reasonable question, if wine evokes for you the image of a wine snob, pinky extended, mouthing fancy talk. Certainly no similar mystique surrounds Pepsi-Cola, iced tea or milk. But wine is different.
It's the only beverage I know that appeals to the senses and the intellect.
If you take the time to look for it, every glass contains a lesson in history, geography, agriculture, botany; sometimes anthropology, religion, psychology and more. There's no reason to be snobbish about wine, and none to fear it. But it's well worth talking about and sharing with friends. Avoid taking wine too seriously. This stuff is supposed to be fun. You don't have to pass a test to enjoy it, and you needn't learn a new language.
The idea behind wine tasting is as simple as this: Slow down. Relax and take the time to think about what you're drinking and to enjoy it with all your senses. (Well, all except hearing. Nobody listens to wine.)
Examine its color. Is it clear or hazy, transparent or opaque?
Take a deep sniff. Does it smell like fruit? Flowers? Road tar or sweat sox?
Got it? Take a drink. Take two. Swish it around your mouth, sensing not only its taste but its texture and weight. Don't worry about looks; you're enjoying yourself.
Put it all together in your head. Think about where it came from. Sip again and enjoy. You won't get all this out of a Pepsi!
Getting Your Nose Into Wine.
Wine doesn't have eyes, ears or teeth, but some say it has a "nose."
I won't say the term is snobbish, but I'd feel uneasy about standing around, glass in hand, chatting about a wine's nose. This one's aquiline, that one's pug, the one over there's had an operation?
For that matter, I'm not too comfortable with the distinction some tasters make between a wine's "aroma," referring to the natural smell it takes from the fruit, and its "bouquet," the complex overtones it may develop with age in the bottle.
Three terms to refer to one sense? It reminds me of the Eskimos, who reportedly have scores of words to define subtleties in snow, from snowball-packing quality to bricks for igloos.
So let's strike a blow for clarity in wine language by agreeing to use plain English here. I'll talk about how a wine "smells," and if I feel the need for synonyms, I might refer to its
aroma or scent. I'll warn you if I find one that stinks. One thing makes common scents: Smell is important to the wine taster. Much of what we think is taste really comes through our noses. If you don't believe it, try to enjoy a wine -- or a meal --the next time you have a bad head cold.
When it comes to smelling, we take a distant second place to dogs and cats. Still, we humans can train our sense of smell, and you don't have to be an expert wine taster to learn to sniff out the differences among wines.
The aroma of Cabernet Sauvignon and the closely related Merlot grape, for example, often reminds me of cedar wood and pine needles mingled with a good fruit smell reminiscent of currants.
Some add hints that wine tasters call "vegetal:" green olives, green peppers, tobacco leaves or grass.
Aging the wine in oak may add touches of vanilla, cinnamon, cloves and almonds. Extended bottle aging may lend a toasty quality and impart earthy scents as variable as mushrooms, old leather, roses and wildflowers.
Other grapes have their own trademark aromas: Zinfandel often evokes berries. Pinot Noir, the fine grape of Burgundy, may recall violets and spice. The pungently floral quality of freshly ground black pepper signals Syrah, the French Rhone grape.
Among whites, Chardonnay recalls crisp, ripe apples and may add notes of butter, coconut, figs and other tropical fruits, particularly if it's aged in oak.
Riesling, the queen of German grapes, may evoke apples, too, and sometimes citrus fruit, canteloupe and pine.
Sauvignon Blanc often shows a grassy smell and sometimes grapefruit. Chenin Blanc reminds me of melons and, occasionally, orange blossoms. A smell of peaches identifies Muscat and Gewurztraminer; the latter may add elusive spice.
Taste: More Than Just Swallowing
"Taste" doesn't mean only what we sense with our mouths.
The words also describes the quality of critical discernment, judgment and appreciation that separates most of us from animals at a trough.
We taste the joy of victory and the bitterness of defeat. We savor life and we sample the flavor of an experience.
Scientists tell us that our taste buds can discern only four basic flavors: Sweet, sour, bitter and salty.
What we think of as taste, however, is a much more complex sensory experience that combines what our taste buds tell us with the senses of smell and touch.
Yes, I said touch. The feel of the wine in your mouth, its sense of lightness or weight, a quality that may range from watery-thin to viscous and oily is very much a part of the experience of tasting wine.
Sourness is a fault in wine if it reeks of vinegar, the sign of a spoiled beverage (fortunately, you'll rarely find it nowadays). In the form of crisp, sharp acidity, however, a sour sensation is a desirable trait, offering a brisk, acidic taste that's as amiable a companion to fish as a squirt of fresh lemon. A wine with too little acid, on the other hand, may seem mellow at first, but it's bland and uninspiring, lacking the verve to stand up to food.
Sour and sweet tastes are mixed in many California Chardonnays, which at their best are crisp, almost dry, with just enough fresh-fruit sweetness to soften the cutting acidic edge.
Finally, sweet dominates the sour in "late harvest" and other dessert- type wines, in which a penetrating sweetness identifies the style, but the sugar is balanced against sharp acid that keeps the wine from cloying.
Wine Vocabulary - All Those Funny Words.
A friendly copy editor came by the other day, as copy editors sometimes do, with a logical question that wasn't easy to answer.
"I don't know that much about wine," she said. "But I have a little trouble relating to something that you say tastes like 'old leather' or 'melting road tar' -- and you seem to like it."
She's got a point.
One of the most challenging things about judging wine -- and telling other people about it -- is that so much of its appeal is to our senses of smell and taste.
Since we humans don't use smell or taste nearly as much, or as effectively, as we do sight, hearing and even touch, we lack a well-defined, precise vocabulary to describe aromas and flavors in terms that mean the same thing to everyone.
It isn't easy to do that accurately, vividly and effectively without drifting into intolerable vagueness, dropping into incomprehensible jargon or using the kind of precious language that makes people think you're a wine snob.
Furthermore, a lot of the terms that most accurately describe frequently occurring scents in wine are not words that we usually associate with edible things. Oak, cedar and pine, for instance. Moss, leaves and grass. Yes, even tar and leather.
(Carrying this to its logical extreme, in 18th century France the aroma of fine Burgundy was more than once likened to raw sewage, to put it relatively delicately. This was intended as a compliment, something that might be difficult to comprehend unless we consider the way the French love strong cheese.)
It's also important to understand that these scents and tastes rarely dominate the wine. Typically they add a small but significant element to a larger pattern, as a colored thread might highlight woven cloth or a French horn's theme add texture to an orchestral chorus.
In other words, the hints of chocolate and coffee in some California red wines and the nuances of coconut, figs and dates in oak-aged Chardonnay don't make the wine taste like a milkshake or fruit salad; they are subtle, often elusive parts of a larger whole.
That "tarry" quality in a California Merlot that puzzled my friend, the editor, is not an unpleasant scent to me but one of great nostalgia, evoking memories of youthful hikes along the edge of country roads on hot summer days.
The French even have a name for it -- gout de goudron -- according to Frank Schoonmaker's Encyclopedia of Wine, which notes that the smell, "far from disagreeable ... is usually one of the characteristics of a fine red wine made from very ripe grapes."
The smell of old leather comes up often in well-aged red wine. I find it pleasant, too, more like fine old books in leather bindings than well-used shoes.
The scents of wine come from several sources. The fruity smell of young wines comes directly from the grapes, with woody and other organic aromas added if the wine was aged in oak.
Fine, aged wines add the most complex (and sometimes un-winelike) scents, which some wine tasterscall "bouquet," as the result of gradual chemical reactions in the wine. Less pleasant changes inodor and taste occur if the wine is poorly or carelessly made or spoils with excess age.
Just for fun, I scanned back over years of my tasting notes and several good wine books to get anidea of the breadth of vocabulary wine tasters have used.
Emile Peynaud's "Le Gout de Vin" ("The Taste of Wine," quoted in Robert M. Parker Jr.'s "Wines of the Rhone Valley and Provence") divided wine aromas into nine principal categories:
Animal odors, smells of game, beef and venison; balsamic odors, smells of pine trees, resin and vanilla; woody odors, smells of new wood of oak barrels; chemical odors, smells of acetone,mercaptan (skunks or natural gas), yeasts, hydrogen sulfide (rotten eggs), lactic and fermentation odor; spicy odors, smells of pepper, cloves, cinnamon, nutmeg, ginger, truffles, anise and mint; empyreumatic (creosotes and oils) odors, smells of creme brulee, smoke, toast, leather and coffee; floral odors, smells of flowers, violets, roses, lilacs, jasmine; fruity odors, smells of blackcurrants, raspberries, cherries, plums, apricots, peaches, figs; and vegetal odors, smells of herbs, tea, mushrooms and vegetables.
Other frequently occurring scents include apples (a characteristic of Chardonnay and Riesling grapes); green olives, green peppers, even asparagus (typical of inexpensive red wines from some cool regions); walnuts and pecans (desirable in Sherry, a flaw in wines oxidized with age); vinegar (a breath is common in Beaujolais, more than a breath is a fatal flaw in any wine); andchalk or steel (reminiscent of licking a clean pebble or knife blade, the trademark of French Chablis and some other acidic Chardonnays).
Young wines are usually simple and straightforward, offering uncomplicated smells of grapes and fresh fruit.
It's bottle age that brings about the chemical changes that provide unusual and (one hopes)
delicious nuances that cry out for descriptive terms.
Learning to Taste by Closing Our Eyes.
It was Monday morning, and the managing editor approached my desk with a gleam in his eye and what I hope was a smile on his face.
He wasn't waving my Sunday column around, but he might have memorized it.
"I will give you $1,000 if you can really smell and taste all those things you said you found in that wine," he said.
"I hope the check's in the mail," I shot back. "I could use the money."
"Apples and grapes," he harrumphed, ignoring me. "Figs. Coconut. Probably old shoes and wood chips."
About that time his boss strolled by, gave us a look and shook his head.
I think my boss was just kidding. He knows wine himself.
He's got a point, though. The complex aromas and flavors that distinguish fine wine are usually subtle and sometimes almost -- but not quite -- as elusive as the emperor's legendary new clothes.
It's not hard to learn to recognize these subtleties, but it takes practice, which makes perfect in wine appreciation as it does with just about anything else worth appreciating.
Bordeaux wine maker Alexis Lichine once said the best way to learn wine is by opening bottles.
I'd add that the best way to learn wine quickly and well is by frequently tasting wines "blind," judging comparatively without knowing what's in the glasses until you've made your notes and announced your conclusions.
Nothing concentrates the wine taster's attention quite as intensely as having someone waiting to rib you mercilessly if you can't tell a Chardonnay from a Chenin Blanc.
Gaze under such circumstances at two near-identical glasses of golden Chardonnay, and it won't take long to discern the nuances of gold, bronze and brass, apples, chestnuts, figs and yes, even coconut in the wine.
I rate the wines for this column blind for another reason: Even the most objective judge will be influenced to some degree by knowing what's in the glass. When you're comparing a $20 nectar against a $3 jug wine, it's a lot easier to be honest if you don't know which is which.
Postgraduate Blind Tasting
So you think it's easy to tell red wine from white?
Try doing it blindfolded sometime.
Some white-wine drinkers who rarely touch red are convinced that the differences between the types are deep and fundamental.
Consider the stereotypes: White wine is light, fruity and refreshing, an anonymous tipple for casual sipping. Red wine is strong, complicated and (although fine for connoisseurs, perhaps) hard to get to know.
Are the stereotypes valid?
Or are the differences overshadowed by the similarities between what are, after all, beverages made from fruit as closely related as red (or blue or purple) and white (or green or golden) grapes?
Prompted by a recent discussion on the subject among several friends communicating with personal computers on the CompuServe Information Service's Wine Forum, I decided to find out by taking the practice of "blind" tasting to its logical extreme.
I usually rate the wines for this column "blind," sampling the week's wine selection from plain,
unmarked glasses poured out of my sight.
The point is to ensure that my objectivity is unmarred by prejudice or preconceived ideas. It's easier to be objective if I don't know know which glass contains the $20 boutique wine and which holds the $2.99 jug variety.
It's easy enough to arrange this kind of tasting: All you need is someone to pour the wine. It doesn't matter if you see what's in the glass.
It's a bit more complicated to compare red and white without looking, as a real (if temporary) loss of vision is required. I achieved the effect by asking my wife to wrap a red bandanna around my head.
I used four moderately priced wines -- two white and two red -- for the test.
I chose two California wines -- a red 1981 Inglenook Vineyards Napa Valley Petite Sirah ($5.49)
and a white 1985 Gundlach Bundschu Sonoma County (Rhinefarm Vineyards) Gewurztraminer ($6.49) --
anticipating that these two wines would display marked characteristics that should be easy to
choose.
To mix things up, I added a white 1985 Collavini Grave del Friuli Pinot Grigio from Italy ($5.79)
and a red 1983 Premiat Dealul Mare Cabernet Sauvignon from Romania ($2.99), expecting them to be
simple, fruity wines that might be more difficult to distinguish without benefit of sight.
The results?
Differences do exist, but they're more subtle than you might expect. I found it fairly easy to tell the red from the white, but it would have been much more challenging without the benefit of quite a few years' tasting experience. As it was, it wasn't easy pegging all four wines to their specific labels.
Here's a summary of the notes I dictated to a tape recorder during the blind tasting.
Glass No. 1 (the Petite Sirah) was easy. Scents of green olives and black pepper and the mouth-filling, fruity and acidic flavor gave away the grape variety in this gutsy, full-flavoredwine, the best wine of the four at a bargain price.
Dry acidity and a hint of oak were the tell-tale signs that Glass No. 3 (the Romanian Cabernet) held the other red wine.
I picked the two remaining glasses as white but misidentified their contents.
Glass No. 2 was obviously white. It could have passed for an inexpensive Rhine wine with a soft, faintly sweet taste. Its musky aroma, reminiscent of overripe canteloupe, wouldn't have been surprising in a Gewurztraminer, but the wine proved to be the Pinot Grigio.
A citrus quality with a faintly bitter aftertaste made clear that Glass No. 4 was white, but, misled by the Italian wine's muskiness, I failed to recognize this wine as "Gewurz;" it lacked the exuberantly spicy quality typical of this flavorful grape in Europe. It was a useful lesson, and at least I salvaged my ego by correctly identifying all the reds and whites.
Our Vinous Debt to France.
France, once once the world's leading wine-producing nation, lost claim to that title when Italy increased its annual production to 2 billion gallons some years ago.
It's not the oldest wine-producing nation, for wine was made around the eastern Mediterranean basin millenia before Caesar divided Gaul into three parts.
Indeed, the French can't even claim undisputed bragging rights as producers of the world's best wines. Vinous competitors around the world, from Italy to California to Australia, would have something to say about that.
None of which takes away from this: Without the contributions France has made, wine as we know it today wouldn't be wine.
Back in the 12th Century, when the English held Bordeaux, they learned to love the local wine, a beverage they called "claret."
Ever since that time, around the civilized world, the standard for fine wine -- the dry, acidic type that marries well with food -- has been based on the French model.
So simple respect for wine history demands that I begin the second part of my brief refresher course in wine tasting -- a country-by-country review of wines from around the world -- with a look at France.
France, which remains second-largest wine producer in the world, produces tiny quantities of some of the greatest and most expensive wines. It also produces huge quantities of vin ordinaire (everyday drinking wine) that's rarely exported.
In the middle there's a good selection of decent, fairly priced table wine that gives a good idea of the debt wine lovers owe to France.
For this week's column I sampled a trio representing three French wine regions: A 1983 Trimbach Gewurztraminer from Alsace ($7.69); a 1982 Jean-Pierre Mouiex Saint-Emilion from Bordeaux ($8.89);and a 1985 Jaboulet-Vercherre "Chassy" Cotes-du-Rhone from the Rhone region ($4.99).
The Gewurztraminer was a typically robust wine of Alsace, a region along the Rhine across from Germany, where the wines must stand up against the sausages and sauerkraut that highlight the region's hearty cuisine.
The Saint-Emilion is a "shipper's" wine, a relatively inexpensive product compared with the more expensive "chateau-bottled" wines made of grapes grown on the wine maker's own property. However, Moueix is one of the best shippers of France, and even his cheap wines are good. This one, from the widely hailed 1982 vintage, was a bargain; I can't imagine a better way to learn for less than $10 what Bordeaux is all about.
If you've ever ordered a pitcher of red wine in a Parisian bistro, you've likely tasted Cotes-du-Rhone. It's an intensely fruity, sharply acidic red wine that goes well with red meat, but it's no mellow sipper. If your tastes run to sweetish White Zinfandel, this one might take some getting used to.
Wine and Fun From Italy
If the wines of France are serious, the wines of Italy are fun.
Italy produces more wine, and makes it in greater variety, than any other nation.
From top to toe of the Italian Postgraduate Blind Tasting (Nov. 29, 1987)
So you think it's easy to tell red wine from white?
Try doing it blindfolded sometime.
Some white-wine drinkers who rarely touch red are convinced that the differences between the types are deep and fundamental.
Consider the stereotypes: White wine is light, fruity and refreshing, an anonymous tipple for casual sipping. Red wine is strong, complicated and (although fine for connoisseurs, perhaps) hard to get to know.
Are the stereotypes valid?
Or are the differences overshadowed by the similarities between what are, after all, beverages made from fruit as closely related as red (or blue or purple) and white (or green or golden) grapes?
Prompted by a recent discussion on the subject among several friends communicating with personal computers on the CompuServe Information Service's Wine Forum, I decided to find out by taking the practice of "blind" tasting to its logical extreme.
I usually rate the wines for this column "blind," sampling the week's wine selection from plain, unmarked glasses poured out of my sight.
The point is to ensure that my objectivity is unmarred by prejudice or preconceived ideas. It's easier to be objective if I don't know know which glass contains the $20 boutique wine and which holds the $2.99 jug variety.
It's easy enough to arrange this kind of tasting: All you need is someone to pour the wine. It doesn't matter if you see what's in the glass.
It's a bit more complicated to compare red and white without looking, as a real (if temporary) loss of vision is required. I achieved the effect by asking my wife to wrap a red bandanna around my head.
I used four moderately priced wines -- two white and two red -- for the test.
I chose two California wines -- a red 1981 Inglenook Vineyards Napa Valley Petite Sirah ($5.49)
and a white 1985 Gundlach Bundschu Sonoma County (Rhinefarm Vineyards) Gewurztraminer ($6.49) -- anticipating that these two wines would display marked characteristics that should be easy to choose.
To mix things up, I added a white 1985 Collavini Grave del Friuli Pinot Grigio from Italy ($5.79) and a red 1983 Premiat Dealul Mare Cabernet Sauvignon from Romania ($2.99), expecting them to be simple, fruity wines that might be more difficult to distinguish without benefit of sight.
The results?
Differences do exist, but they're more subtle than you might expect.
I found it fairly easy to tell the red from the white, but it would have been much more challenging without the benefit of quite a few years' tasting experience. As it was, it wasn't easy pegging all four wines to their specific labels.
Here's a summary of the notes I dictated to a tape recorder during the blind tasting
Glass No. 1 (the Petite Sirah) was easy. Scents of green olives and black pepper and the mouth-filling, fruity and acidic flavor gave away the grape variety in this gutsy, full-flavored wine, the best wine of the four at a bargain price.
Dry acidity and a hint of oak were the tell-tale signs that Glass No. 3 (the Romanian Cabernet) held the other red wine.
I picked the two remaining glasses as white but misidentified their contents.
Glass No. 2 was obviously white. It could have passed for an inexpensive Rhine wine with a soft, faintly sweet taste. Its musky aroma, reminiscent of overripe canteloupe, wouldn't have been surprising in a Gewurztraminer, but the wine proved to be the Pinot Grigio.
A citrus quality with a faintly bitter aftertaste made clear that Glass No. 4 was white, but, misled by the Italian wine's muskiness, I failed to recognize this wine as "Gewurz;" it lacked the exuberantly spicy quality typical of this flavorful grape in Europe.
It was a useful lesson, and at least I salvaged my ego by correctly identifying all the reds and whites.
Germany: Hard Words, Easy Wine.
Nothing in the world of wine is much more daunting than the German label.
The language is polysyllabic and agglutinative, and not only that, it uses jaw-breaking words that are hard to read even if they aren't printed in old-fashioned Gothic type.
But it would be a shame to let a few big words keep us from discovering German wine.
Because it almost invariably has an edge of sweetness and its alcoholic content is typically low, German wine can be an attractive change of pace from dry, acidic table wines. Don't expect a wine that tastes like Kool-Aid, though. At its best, German wine balances natural sweetness with tart acidity that keeps the taste from cloying.
Because Germany's Rhine and Mosel valleys are among the world's most northerly wine-producing regions, growers run a constant race against the weather. Long but cool summers allow an extended growing season, with the harvest sometimes coming as late as November. Grapes ripen slowly in this climate, acquiring subtle qualities from the soil.
In a good year, such as 1983 or 1985, fully ripened grapes produce lush, succulent wine with exceptional complexity and finesse
In poorer vintages, though, the grapes don't ripen well and wine makers must add sugar to the green, acidic juice. It's not a formula for excellent wine.
Most German wines are submitted to a government panel for tasting and laboratory tests to verify their origin and sugar content.
Wines that pass the examination receive the designation "Qualitatswein eines bestimmten Anbaugebietes," often shortened to "Qualitatswein" or "QbA."
The finest, made from grapes so ripe that no additional sugar is needed, receive the designation "Qualitatswein mit Pradikat" or "QmP." These wines are further categorized, in order of increasing sugar content and (usually) price, as "Kabinett," "Spatlese," "Auslese," "Beerenauslese" or"Trockenbeerenauslese."
German wine seems like it ought to be a natural for Americans with its light, sweet flavor and low alcohol content. Don't let the label scare you. Give it a try!
Spain: A Delicious Secret
Spain makes nearly as much wine as Italy and France, but the natives drink it right up and export little, so we don't see much Spanish wine in this country.
This is a shame, because the wines of Spain can be startlingly good, combining the finesse and character of France's finest with a sunny, Mediterranean quality like that of Italy.
On the other hand, it's a delicious secret for wine tasters in the know, because the laws of supply and demand have kept most Spanish wines in the bargain range.
Spanish sparkling wines from Freixenet (particularly its Cordon Negro in a Darth Vader-black bottle) and Codorniu (whose "English Cuvee Brut Clasico" is my favorite Spanish sparkler) are gaining deserved popularity while remaining in the modest $6 range.
Sherry, a British mispronunciation of "Jeres," the town near Seville from which it's shipped, has been popular with Anglo-Saxons for centuries but still remains cheaper than currently-chic Port from Portugal.
I like Spanish red wines best. Good ones, drunk young, are as bright and refreshing as grape juice. Aged in wood and then in the bottle, they add a spicy savor that's hard to match at any price.
Rioja, in north-central Spain, above Madrid, became home to emigrant French wine makers more than 100 years ago; they applied their traditional skills to their new country's native grapes to create a new wine with a familiar accent.
Among Rioja labels usually available in this area are the products of Marques de Caceres, Marques de Riscal, Domecq and Olarra. Olarra, a relatively new and very large bodega (winery), has made a considerable effort to capture an American market. Its wines, if rarely outstanding, are consistently good and have beenavailable here at remarkably low prices.
The USA: Fine Wine from the Melting Pot
Like our nation itself, the wines of the United States are the bold and lusty product of an international melting pot.
We have France to thank for the dry acidic style of table wine that places a premium on subtle complexity. Our finest grapes, too, are French: The Cabernet Sauvignon, Chardonnay, Merlot, Pinot Noir and more.
Italy taught us the joy of wine as an ideal companion with food. Germany contributed the Riesling grape and a taste for sweeter wines. The story of American wine is told in a babel of accents and bears a United Nations of names.
From Count Agostin Haraszthy, who got the California wine industry on its feet a century ago, to Andre Tchelistcheff, who did as much as any man to professionalize U.S. wine making since World War II, the list goes on:
Paul Masson and Charles LeFranc were early luminaries. Later came Martini, Parducci, Sebastiani and more, all names we recognize on labels today.
Robert Mondavi convinced his peers that California could make wine to compete with the best of France. Mondavi's Napa Valley neighbors, Joe Heitz and Joseph Phelps, Bill Hill and Bernard Portet, among dozens of others, made great wines to prove the point.
Ernest and Julio Gallo taught the world how to sell good wine. Along the way, with much of the activity in California but also bursting out across most of the 50 states, the old ways have gradually blended into a new, all-American style. Rich fruit, ripe grapes, deep, powerful aromas and flavors: These qualities characterize American wine at its best.
Sparkling Wine: The Spirit of Festivity.
The French have been arguing for decades, without perceptible success, to persuade Americans to stop calling our sparkling wine "Champagne."
That honored name, they say, should be reserved for the fine, sparkling wines first made in the French region of the same name, the neighborhood around Rheims, not far east of Paris.
According to legend, a blind French monk named Dom Pierre Perignon had the first taste of Champagne, sometime around 1700, when he stumbled upon a cask in which an accidental fermentation had occurred, tasted the bubbly product and exclaimed, "I am drinking stars!"
It's a harmless tale, even if it probably isn't true (although the original Dom Perignon, whose name now adorns a pricey French product, probably did play a key role in the development of Champagne as we know it today).
Not only that, but it's a far more poetic turn of phrase than the epigram attributed to Cole Porter, who allegedly sipped Champagne and observed, "It tastes as though my foot's asleep."
Champagne (like other sparkling wines) tends to inspire flights of fancy. I've never been quite sure whether this effect is purely psychological -- it is, after all, a festive drink that invokes a celebratory mood -- or perhaps comes about because they carbon dioxide bubbles somehow speed alcohol to the user's brain.
Be that as it may, sparkling wine is a popular drink, one of the few wine categories that showed increased sales in the United States amid a general decline in wine and spirits sales last year.
In one of the most interesting developments of recent years, a number of French Champagne makers -- without for a minute relenting in their campaign to reserve the name for themselves -- have opened wineries in California and started making sparkling wine.
Moet et Chandon (makers of the aforementioned Dom Perignon) came first, early in the 1970s, with a sleek, modern winery in Napa County near Yountville. The wine, Domaine Chandon, is one of the best California sparklers, and the winery -- with its associated four-star restaurant -- has become a must stop for Northern California wine-country tourists.
Piper-Heidsieck of France owns Piper Sonoma Cellars, makers of another excellent California sparkler.
Deutz, another French firm, now makes Maison Deutz in Santa Barbara County, Calif., another wine with excellent credentials; ditto for Domaine Mumm Cuvee Napa, wholly owned by a famous French house. The French aren't the only ones making sparkling wine, of course.
If your experience is limited to the $3 domestic brands that somebody picks up at the drugstore when there's call for an impromptu celebration, I'd suggest widening your horizons.
Although French Champagne can be expensive -- most basic lines start below $20 and can run up to $100 for a couple of chic cuvees -- quality California sparklers abound in the $10-to-$15 neighborhood.
Spanish sparkling wine is generally less and can be quite good, and the Italian Asti Spumantes, fizzy wines made from Muscat grapes, are refreshing if your taste runs to sweeter wine.
Tasting on the Wheel.
We actually smell most of the things that we think we taste, or so the scientists say.
Our poor taste buds can discern only four flavors -- sweet, sour, salt and bitter -- while our noses are capable of distinguishing thousands of subtle variations.
There's nothing like a summer cold or allergy attack -- with its attendant loss of the sense of smell -- to bring this theory out of the laboratory into the real world.
If you like wine and catch a cold, you're well advised to switch to soft drinks, pure spring water or something equally undemanding until the sniffles have passed and you can again enjoy the olfactory nuances that make wine something special.
The wines of the world offer thousands of scents in their almost infinite variety. I must have smelled a few hundred things in wine myself, ranging from the commonplace (grapes and fruit) to the off-the-wall (sawdust and asparagus) and the disgusting (dirty socks and wet dog fur).
As an aid to novice wine tasters -- and experts too -- the wine scientists at the University of California at Davis, one of the nation's leading wine-making and grape-growing schools, came up years ago with something called the "aroma wheel." The oenologists at Davis consulted with scores of wine lovers and wine tasters to list all thedescriptive terms they could imagine for the smells of wine. Then they organized them, categorizedthem, eliminated all that seemed ambiguous or less than clear, and ended up with a list of 12major categories of wine smells, subdivided into 29 subcategories and in 94 specific terms.
The original "wheel" was so called because it was displayed as a circular table, with relatively similar smells placed close together around its circumference. (Colored plastic laminated copies of the wine aroma wheel may be obtained from A.C. Noble, Dept. Vit. & Enology, Univ. California, Davis, Calif 95616 [email ]. The wheel can be viewed on the UCDavis Website.)
You don't need a wheel to get rolling, however: The information is just as useful in the form of a list, starting at noon and moving around the clock from "fruity" through "nutty" and "earthy" around to "floral," "spicy" and back to fruity again.
If you want to get more out of your wine, try your next tasting session with the list at hand, scanning the categories in search of the exact word to describe what you're smelling.
I think you'll be surprised to see how a glance at the "wheel" helps your thoughts snap into focus. I've edited the following list somewhat to save space, leaving out some of the more obscure and technical terms, but you'll find all the usual aromas rounded up here:
FRUITY: Citrus -- grapefruit, lemon; berry -- blackberry, raspberry, strawberry, black currant (cassis); tree fruit -- cherry, apricot, peach, apple; tropical fruit -- pineapple, melon, banana; dried fruit -- strawberry jam, raisins, prune, fig.
VEGETATIVE: fresh -- stemmy, cut green grass, bell pepper, eucalyptus, mint; canned-cooked --green beans, asparagus, green olive, black olive, artichoke; dried -- haw-straw, tea, tobacco.
NUTTY: walnut, hazelnut, almond.
CARAMELIZED: honey, butterscotch, butter, soy sauce, chocolate, molasses.
WOODY: vanilla, cedar, oak, smoky, burnt toast, charred, coffee.
EARTHY: dusty, mushroom, musty (mildew), moldy cork.
CHEMICAL: petroleum -- tar, plastic, kerosene, diesel; sulfur -- rubbery, garlic, skunk, cabbage, burnt match, wet wool, wet dog; papery -- wet cardboard; pungent -- acetic acid (vinegar); other-- soapy, fishy.
PUNGENT: hot -- alcohol; cool -- menthol.
MICROBIOLOGICAL: yeast, sauerkraut, sweaty, horsey, "mousey."
FLORAL: orange blossom, rose, violet, geranium.
SPICY: cloves, black pepper, licorice, anise.
Use the "wheel" as a guide when you're tasting wine for fun, and I think you'll be surprised to see how well this list of descriptive terms will help you recognize those elusive characteristics.
--------------------------------------------------------------------------------
remember two simple rules -- (1) think about wine, and (2) keep opening bottles -- you'll soon be
on your way to expertise and a lifetime of enjoyment. Good luck, and good wine!
The Use of Sulphur Dioxide (SO2) in Winemaking
When Nature ferments grapes, or any other fruit for that matter, wine is not the end product. Instead, unpleasant concoctions containing vinegars, mercaptans and other substances are formed, with the final end being water and assorted solids and gases. Although most good winemaking involves interfering with Nature as little as possible nonetheless we need to steer her a bit, and in fact completely stop some natural processes at just the right moment.
An indispensable ally of the winemaker in achieving these things is sulphur dioxide. We will refer to it by its chemical formula, SO2. In this article we will be investigating how to use SO2 to do the following things for us: inhibit wild and spoilage yeasts and unwanted bacteria (this can include the malolactic bacteria at sufficiently high SO2 levels); help prevent oxidation and preserve fruity flavour and freshness in wine.
Sourcing SO2
SO2 is a pungent, choking gas which is somewhat soluble. The most practical source for the home winemaker is the salt, potassium metabisulphite, which is 57% SO2 Since you can detect SO2 when you smell a sample of potassium metabisulphite it is evident that the solid decomposes easily. This happens on contact with carbon dioxide and moisture in the air. Keep your container of potassium metabisulphite tightly closed to minimize this problem. In any case, you probably shouldn’t keep the stuff around for more than a year before buying fresh.
“Campden” tablets are made of potassium metabisulphite. Each tablet, when fresh, contains 0.44 grams of it. However, if they are old, a lot of the SO2 will have been lost and their effects will be unreliable. You’re better off to use bulk potassium metabisulphite. It’s cheaper too.
Sodium metabisulphite is also a source of SO2 but probably should be used only for equipment sterilizing purposes, not in must or wine. For one thing, many people avoid sodium in their diets, for another, the presence of potassium ions in wine is more useful than sodium.
Sometimes an old fashioned winery will burn a sulphur stick in empty barrels to keep them sterile. Under no circumstances should the home winemaker ever do this. The presence of any elemental sulphur, such as might drip into the barrel will lead inevitably to the formation of the dreaded hydrogen sulphide. In the winemaking business, -ite sulphur compounds are friendly, -ides are deadly enemies.
Under a very few circumstances, solid potassium metabisulphite may be used directly. For instance if you decide to add SO2 to red grapes before crushing, a scant ¼ teaspoon sprinkled on a 36 pound lug of grapes will give you about what you need—somewhere around 30 to 40 parts per million SO2.
Don’t do this with white grapes or when using red grapes to make a rosé. When you press, the SO2 will wash off into the juice in uncontrollable amounts and you will likely have far too much in the free run, and next to none in the pressed portion.
The 10% Solution
A much better way to get your SO2 is from a 10% solution of potassium metabisulphite in water. For instance, you could add water to 1 pound of potassium metabisulphite to make a total volume of 1 imperial gallon. Or, if you prefer metric, add enough water to 100 grams of potassium metabisulphite to make up a total volume of 1.00 litres. Fresh 10% solution is 5.7% SO2.
A commonly used unit of measurement for SO2 in must or wine is “parts per million” or “ppm”. 1 ppm is the same as 1 milligram per litre. I will use ppm.
For example, if you add 2.4 millilitres of 10% potassium metabisulphite solution to 1.0 imperial gallons of wine you will be adding 30 ppm SO2. If you have a 19.2 litre carboy to which you wish to add 20 ppm SO2, multiply 0.35 by 19.2 to get an SO2 addition of 6.7 mL of 10% solution. Consider making up your own spreadsheet giving SO2 additions for your own sizes of barrels and carboys.
Putting SO2 to good use
You might hear a commercial winemaker tell you that she “doesn’t use any SO2 at all until after the primary ferment is complete, particularly with white wines.” Such a winemaker knows the complete history of her grapes—exactly where they came from and how they were handled. She undoubtedly also has elaborate handling equipment—must coolers, inert gas covered tanks and all the rest. You should know a lot about what you’re doing before you decide to postpone adding SO2 until some middle point in the winemaking process.
Let’s start with red grapes. You need to suppress any bacteria and wild yeasts they may have picked up, prior to inoculating with a selected yeast culture. If you try to depend on wild yeasts, they will likely die before all the sugar is fermented out, leaving you with a sticky problem or worse. Vinegar bacteria can produce an undesirable amount of ethyl acetate in the early part of the fermentation if not checked.
You probably bought the grapes by the pound and can assume around 5 litres of finished wine from each 20 pounds. Addition of 2.7 millilitres of 10% potassium metabisulphite solution for each 20 pounds works out to 30 ppm SO2. If the grapes are in reasonable shape, this should do the job for you. If your grapes are in perfect shape and the pH is low enough, you can do with less. We will deal with pH considerations later.
If you are planning to have a malolactic ferment, or ML, happen at the same time as the sugar ferment, don’t add the ML culture until the sugar ferment is well underway. By that time enough of the SO2 will have gone so that the ML bacteria can multiply and flourish. Alternatively keep your SO2 addition down to, say, 20 ppm. We’ll talk more about ML when we discuss white wines.
If you are concerned about excessive mould, possibly accompanied by traces of vinegary smells, increase the SO2 addition to 50 or 60 ppm or in extreme cases even more.
The SO2 you add will also lead to production of small quantities of glycerol in the early part of the ferment. This is generally desirable.
When you make white or rosé wine the situation is a bit different. Grape skins contain phenols. These add flavour and colour to wine. They can also contribute astringency, bitterness and browning. These things are of more concern in whites and rosés than in red wines.
SO2 can contribute to phenol extraction from the skins and this is another reason it shouldn’t be added to a white or rosé until after the pressing has been done. However, the addition should be made promptly since white must quality suffers from oxygen absorption from the air. As soon as you have pressed, you have an accurate measure of your yield and can thus calculate the SO2 addition more precisely.
How much SO2?
How much to add depends on a number of factors. What was the condition of the grapes? What is the pH? (We shall see later, how SO2 is more effective at lower pH). Are you planning on putting the wine through a malolactic ferment? Is the juice intended for making a champagne method sparkling wine?
30 ppm SO2 for juice from sound fruit with a pH of 3.4 or so, and destined for a regular wine should be fine.
If you hope to have a malolactic ferment happen along with the sugar ferment, you likely have a higher acid Chardonnay, or something, say around pH 3.2. Smaller SO2 additions are okay here—say 20 ppm. Malolactic bacteria won’t work at levels higher than around 15 ppm, but by the time you add an ML culture, much of the SO2 will have been used up.
Juice destined for Champagne method wine will probably have a low pH, close to 3.0, say. You are going to want to have a malolactic ferment occur. If the grapes were perfect, you might get away with no SO2 at all until the first racking. This however is a bit nerve-wracking, like having a tooth filled without anaesthetic. The danger of some undesirable oxidation of the must is there, so better to go with 10 ppm SO2 or so.
At the other end of the scale, juice from grapes with a lot of mould, possibly with some vinegary smells, should have 50 to 60 ppm SO2 or even more added. Who knows—maybe you have lucked on to some botrytised Riesling or Semillon and plan a serious dessert wine. Botrytised grapes may require 100 ppm SO2 or even more for adequate protection.
How about frozen or sterile packaged musts? With white or rosé juice, you can either trust the shipper to tell you how much SO2 was added, or you can test and make additions accordingly.
It is difficult to test reds for SO2, because the red colouring matter interferes with the chemical reaction involved in the test and also makes it difficult to see the colour change involved. You pretty well have to trust the information on the shipping label. The fact that testing reds for free SO2 is difficult makes it imperative that you keep an accurate record of all SO2 additions in order to be able to estimate the situation at any given time.
The next time you are going to consider adding SO2 to the wine is at the first racking. In most cases, this will be after the sugar fermentation is complete and the new wine is dry.
If you want to stop active fermentation to retain residual sugar, don’t try to use SO2 as your main tool. A vigorous ferment of a strong yeast will laugh at you and carry right on to the end. Selected combinations of racking, fining, chilling and filtering are the way to go. SO2 will be involved, but only as it would be normally used in conjunction with these other processes.
Stifling Oxidation
An important reason for adding SO2 when you rack is to avoid oxidation. It does this in three main ways.
-
When you smell a wine that is oxidized, the chemical you are smelling is acetaldehyde. SO2 combines with acetaldehyde to form a stable compound.
-
When there is oxygen around, SO2 itself becomes oxidized before phenol compounds in the wine do, and so acts as an oxygen scavenger.
-
SO2 suppresses the activity of enzymes that cause browning and other problems.
|
Chart showing typical relative amounts of free and bound SO2 |
So, when you add SO2 it doesn’t all hang around. Lots of it gets used up doing these various jobs for you and becomes “bound”. The remainder remains “free”. The bound portion consists of two parts. One part is made up of irrevocably bound compounds with aldehydes and proteins. The other part is made up of less stable compounds. These can partly turn back to the free form when the existing amount of free is lowered, or even if temperature is increased. This free portion also consists of two parts: one is relatively inactive bisulphite and the other, smallest of all the segments, as shown in the accompanying chart is molecular This is the crucial active portion and its size depends both on pH and the total amount of free SO2.
It is worth noting at this point that in the early stages of a wine, when the total SO2 additions are less than 50 ppm or so, roughly half of further additions remains free and half immediately becomes bound. Later, when total additions are above about 60 ppm, most of any further addition remains as free. This knowledge gives us further reason to keep good records of SO2 additions, particularly in the case of reds, where direct measurement of free SO2 is not reliable.
Testing for Free SO2
The test procedure that follows works well only for white or rosé wines. Some of the colouring matter in red wines reacts to the test chemicals in the same way as SO2 making the results pretty well meaningless.
It should be noted that SO2 testing kits may be available at your local winemaking supply shop. Since they will contain all the necessary ingredients, instructions and measuring vessels, you will save yourself substantial effort by buying one. What follows assumes you wish to put together your own kit.
You will need the following chemicals, which you might need some help with. The chemistry teacher at your local high school might be receptive to a contribution to his or her science department’s petty cash fund.
0.02 molar iodine solution: Accurately weigh out 2.54 grams of iodine. Roughly weigh 5 grams of potassium iodide. Add a few millilitres of distilled water, barely enough to cover the chemicals, and agitate until the iodine is completely dissolved. This may take a bit of time. Finally, add enough distilled water to make an accurately measured 1.00 litres of solution.
Dilute sulphuric acid: Add about 250 millilitres of concentrated sulphuric acid to about 750 millilitres of water. Unless you have previous experience handling sulphuric acid, don’t even think of doing this dilution yourself.
Starch solution: Add about 1 gram of starch to about 100 millilitres of water. Stir and bring to a boil then cool.
To do the test: first fill a clean dry 6 or 10 mL syringe with the iodine solution. Next, accurately measure out 50 mL of the wine to be tested. Add 1 mL or so of starch solution and about 10 mL of dilute sulphuric acid.
Immediately start adding iodine solution to the sample, swirling it as you go. You will note a purple-black patch which disappears as you swirl. As soon as the purple colour persists, stop adding iodine, and note how many mL you’ve used. Multiply this by 12.8 to give you the number of ppm of free SO2 in the wine.
Testing for Total SO2
You will need some 10% sodium hydroxide solution in addition to the chemicals required for the free SO2 test. To make this up, add enough water to 10 g of solid sodium hydroxide to bring the volume up to 100 mL. Great accuracy here isn’t necessary. Mix thoroughly.
To do the test: Accurately measure 20 mL of wine and put it in a narrow necked container such as an erlenmeyer flask. Add roughly 25 mL of 10% sodium hydroxide solution. Immediately cover the container and allow to sit for 15 minutes. Fill a clean dry 6 or 10 mL syringe with 0.02 molar iodine solution. At the end of the 15 minutes, add 10 mL dilute sulphuric acid along with about 1 mL of starch solution to the sample and immediately start adding iodine solution. Stop when the purple colour persists. Note the volume of iodine solution used in mL, and multiply by 32. This is your total SO2 in ppm.
Stability of Chemicals.
The dilute sulphuric acid and 10% sodium hydroxide solutions are very stable and will last for years. The sodium hydroxide should be stored in high density plastic in preference to glass. The starch solution will get mouldy. It should be replaced as soon as the slightest bit of discolouration occurs. Iodine is highly volatile. The iodine solution should be in as small a glass container as is convenient, and kepttightly closed and in a cool place. An alternative method of managing the iodine is to make up a 0.20 molar stock solution (10 times working strength). From time to time make up as much working strength (0.02 molar) solution as you will need for a month or so by diluting one volume of the 0.20 molar stock solution with 9 times that volume of distilled water.
Adding SO2 at racking
When racking red wines, depending on pH, the addition of from 20 to 30 ppm SO2 each time should do the trick nicely. For the first couple of rackings, when the total SO2 added since the beginning is less than 50 ppm or so, about half of what you add immediately gets bound, leaving half as free. After your total additions over the life of the wine add up to around 60 ppm or more, most of any additional SO2you add remains as free.
Be sure to pour the SO2 solution into the bottom of the receiving container first and then rack the wine. This way the SO2 is around all the time to suck up unwanted oxygen.
If you have started a malolactic ferment as well and you are not certain it has completed, you could go with less SO2 at racking—maybe 15 ppm, maybe only 10. In this case, your pH is likely to be pretty low anyway and as we’re going to see later, that makes the SO2 much more effective.
You are, of course, keeping a good record of your SO2 additions, aren't you? A reasonable rule of thumb for red wines is to keep the total addition of SO2 from crush to bottling at less than 150 ppm.
With white or rosé wines, test before racking, and add enough SO2 to bring the free up to 20 or 30 ppm.
Once again, if there is a malolactic ferment involved and/or you are going to do a bottle ferment later, for champagne method sparkling wine, you want to keep the SO2 down. Since under these conditions, the pH is going to be low, you are probably okay adding only 10 ppm or so.
A reminder about racking techniques is in order here. Always make sure your syphon tube is down to the bottom of the receiving container. Don’t splash the wine. If you trying to get away with minimal SO2 and you have a carbon dioxide cylinder, purge the receiving container of air with CO2 before adding SO2 and racking.
SO2 and pH
I have made several references to the connection between the effectiveness of SO2 and pH. It is about time to explain how this works.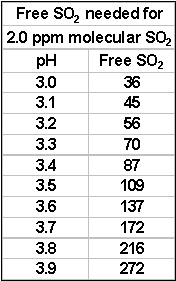
What is really protecting your wine is molecular SO2.
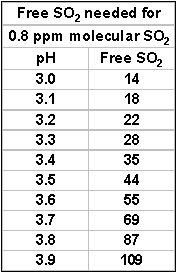 When you add SO2, depending on circumstances, some of it immediately becomes bound. What remains is called “free” and is in two parts. The larger, and relatively ineffective free part is “bisulphite” (HSO3-). The smaller part of the free is the active molecular SO2. The amount of molecular SO2 in your wine depends both on the level of free SO2 present as well as pH. For instance at pH 3.2 the amount of free SO2 for 0.8 ppm molecular SO2 is 22 ppm. At pH 3.5, you will need 43 ppm free – essentially double. In most situations, 0.8 ppm molecular SO2 during bulk storage and at bottling will provide you with adequate protection from oxidation and bacterial action. This includes prevention of ML bacteria as well—important if you’ve blended ML affected wine with non-ML affected and require stability. It is important to remember that the amount of free SO2 in the wine depends on three things: how much you added, how much was present before the addition and how much of your addition promptly becomes bound. In the case of whites and rosés, the best thing to do is a free SO2 check. In the case of reds, you need to do some good estimating, based on previous SO2 additions as mentioned elsewhere in the article.
When you add SO2, depending on circumstances, some of it immediately becomes bound. What remains is called “free” and is in two parts. The larger, and relatively ineffective free part is “bisulphite” (HSO3-). The smaller part of the free is the active molecular SO2. The amount of molecular SO2 in your wine depends both on the level of free SO2 present as well as pH. For instance at pH 3.2 the amount of free SO2 for 0.8 ppm molecular SO2 is 22 ppm. At pH 3.5, you will need 43 ppm free – essentially double. In most situations, 0.8 ppm molecular SO2 during bulk storage and at bottling will provide you with adequate protection from oxidation and bacterial action. This includes prevention of ML bacteria as well—important if you’ve blended ML affected wine with non-ML affected and require stability. It is important to remember that the amount of free SO2 in the wine depends on three things: how much you added, how much was present before the addition and how much of your addition promptly becomes bound. In the case of whites and rosés, the best thing to do is a free SO2 check. In the case of reds, you need to do some good estimating, based on previous SO2 additions as mentioned elsewhere in the article.
The level at which molecular SO2 can be detected by the human senses is about 2.0 ppm. This is also the level which is needed for maximum protection of your wine. This is particularly true in the case of sweet, and most notably, botrytised wines
Using Potassium Sorbate
Sometimes one wishes to finish a wine with some residual sugar left—Riesling, Gewürztraminer, Muscat Canelli and Chenin Blanc are among the grapes that lend themselves particularly well to this. In order to prevent renewed fermentation after ferment has been stopped,00 to 250 ppm potassium sorbate is often used. The effectiveness of potassium sorbate is pH dependent. To get close to the same effectiveness from a given dose of potassium sorbate would require around 55 ppm of free SO2 at pH 3.6 as opposed to only 28 ppm at pH 3.3.
It is essential when using sorbate to have effective SO2 levels high enough to prevent a malolactic ferment from happening. If ML occurs in the presence of sorbate, a peculiarly revolting geranium-like smell is produced for which, alas, there is no remedy. The wine is a goner.
Bottle Rinsing with SO2
I often find it useful to use an SO2 bottle rinse when I am bottling. The rinse solution is 50 mL of 10% potassium metabisulphite solution made up with water, to about 750 mL in a winebottle. I have tested the effect of this several ways, and consistently find that after rinsing, and draining the bottles for about a minute, the free SO2 added is close to 8 ppm. Curiously, this is true for both 750 mL and 375 mL bottles. This is a useful way of adding a touch of SO2 at bottling time, particularly if the carboy you’re bottling has a bit of sediment and you don’t wish to stir it , or subject it to one more racking.
The use of SO2 started with the Romans, and I’m sure there isn’t a self respecting winery in the world today that gets away without it
POTASSIUM SORBATE
Most home winemakers have experienced refermentation of off-dry wines after bottling. This can be very annoying, as it can cause corks to blow out; also, the resultant fizzy wines are yeasty and not always to one's taste. Uncorking the wine and letting it continue its ferment to dryness in a carboy is an unnecessary waste of time, so the usual way of preventing bottle refermentation from happening is to introduce potassium sorbate (sorbate) before bottling. Understand that sorbate does not kill the yeast; it merely inhibits renewed yeast activity under the correct conditions.
Many winemakers use Wine Art's, R.J.Spagnols' or Brewhaus wine conditioners which contain sorbate. Unfortunately, they contain a standard amount of sorbate that is not always sufficient to prevent renewed fermentation. It is probably better to use reserve grape juice or sugar as a sweetener or if there is insufficient unfermented sugar in the wine and add sorbate for stability.
Sorbate addition is dependent upon several interdependent criteria:
Wine pH;
Concentration of free SO2;
Percent alcohol by volume;
Concentration of sorbate; and
Viable yeast cell concentration.
While it is virtually impossible for winemakers to be able to determine the viable yeast cell concentration, they can rest assured that the probability is that there will be some viable yeast cells in off-dry wines. Therefore, the other criteria become very important and are much more easily determined.
Assuming that proper levels of free SO2 are maintained and the pH's are within the desired ranges, sorbate additions can be determined by the estimated alcohol of the wine. The following table is based upon the percentage of alcohol in the wine:
| % alcohol | sorbate addition |
| 10 | 0.20 g/l |
| 11 | 0.17 g/l |
| 12 | 0.135 g/l |
| 13 | 0.10 g/l |
| 14 | 0.07 g/l |
As can be seen, the amount of sorbate decreases as the alcohol level increases. This may be due to two reasons: 1) At the lower alcohol levels, there May be a greater volume of viable yeast cells; and 2) The higher alcohols may have an inhibiting effect on refermentation.
Many winemakers hesitate to use sorbate because of the bubble-gum like aroma and taste it can impart. However, if used properly, sorbate can be very effective without the aroma and taste it can impart. Average threshold levels for detecting sorbate are about 0.182 g/l, so with the proper amount free SO2 and careful measurement of sorbate, the aroma and taste should not be able to be detected by the majority of people. Do not use it in any wine that has undergone MLF; if the MLF recurs, the resultant unpleasant odour will be geraniol, a geranium-like smell.

The Home Winemakers' Wine Lab
| 1.) The Hydrometer | 5.) Sulfite Titrets |
| 2.) The Acid Test | 6.) Balance |
| 3.) The pH Meter | 7.) Chromatography |
| 4.) The Thermometer |
If you are going to be a serious home wine maker than you need to acquire a few piece of equipment and test kits, Above are listed the basic pieces you will need. Only the ph meter will set you back a bit. Most of the items you will need are fairly inexpensive. Don't be afraid to use them. With a little a bit of practice you will be performing tests on your wines like a real wine maker from Napa Valley.
If you want to analyze what is right, or wrong, about your wine, you need to do some science. This can be very simple, or a little complicated, or even ridiculous. For the amateur, there are a few options that help to avoid the extremes. This means we will not be trying to emulate a research lab, but rather use readily available equipment and simplified techniques to discover what we can do to improve our wine. Your home laboratory, which may be as simple as a corner of your winemaking bench, requires a few basics. In broad terms these are measuring equipment, cleaning agents, and records of results.
THE HANDY HYDROMETER
 The hydrometer is the first, and most indispensable, measuring instrument in your home lab. This is a simple device that measures the relative density - or "specific gravity," usually contracted to "SG" - of a liquid. This figure is important for determining, first, how much sugar is contained in the juice (and therefore what the alcohol yield should be) and, finally, when fermentation has reached completion. The hydrometer consists of a thin sealed glass tube with graduated values marked on it, usually in the form of a strip of paper inside the tube, held in place with a spot of glue. The tube has a bulbous, weighted bottom. This makes it float upright, but partially submerged, in a liquid (in our case, wine must). The method is to place the hydrometer in a "testing jar," which is actually a glass or plastic cylinder sealed at the bottom, containing the wine or must to be tested. Ideally, the liquid should be at 60° F when the reading is made. In rough terms, 0.001 should be subtracted from the reading for each 10° F (5° C) below 60° F (15° C), and 0.001 added for each 10° F above 60° F. Since the density of a liquid changes with temperature (density decreases as the temperature rises), the readings will be incorrect if the wine must is at a temperature different from that for which the hydrometer is calibrated. In this case, if the wine must is at 70° F (21° C), a reading of 1.085 actually means a value of 1.086. It should be noted that some hydrometers may be calibrated at a different temperature (for example, 68° F); before applying a correction factor, ensure that you know what the calibration temperature is (it should be marked on the hydrometer itself).
The hydrometer is the first, and most indispensable, measuring instrument in your home lab. This is a simple device that measures the relative density - or "specific gravity," usually contracted to "SG" - of a liquid. This figure is important for determining, first, how much sugar is contained in the juice (and therefore what the alcohol yield should be) and, finally, when fermentation has reached completion. The hydrometer consists of a thin sealed glass tube with graduated values marked on it, usually in the form of a strip of paper inside the tube, held in place with a spot of glue. The tube has a bulbous, weighted bottom. This makes it float upright, but partially submerged, in a liquid (in our case, wine must). The method is to place the hydrometer in a "testing jar," which is actually a glass or plastic cylinder sealed at the bottom, containing the wine or must to be tested. Ideally, the liquid should be at 60° F when the reading is made. In rough terms, 0.001 should be subtracted from the reading for each 10° F (5° C) below 60° F (15° C), and 0.001 added for each 10° F above 60° F. Since the density of a liquid changes with temperature (density decreases as the temperature rises), the readings will be incorrect if the wine must is at a temperature different from that for which the hydrometer is calibrated. In this case, if the wine must is at 70° F (21° C), a reading of 1.085 actually means a value of 1.086. It should be noted that some hydrometers may be calibrated at a different temperature (for example, 68° F); before applying a correction factor, ensure that you know what the calibration temperature is (it should be marked on the hydrometer itself).
To obtain the reading, the hydrometer is floated (not dropped into) the liquid. It is important to handle the hydrometer with reasonable care, since it is somewhat fragile. The hydrometer is then given a couple of quick twists between the fingers to dislodge any bubbles (which would otherwise affect its buoyancy, again leading to inaccurate readings), and then viewed at the point where the stem breaks the surface of the liquid. The scale marked on the hydrometer will give the specific gravity. It should be noted that the liquid actually forms what is called a "meniscus" at the edges, where surface tension causes it to climb slightly above the level. The reading should be made at the point where the surface is penetrated, not at the higher level of the sides.
Many hydrometers on the market, especially professional ones, use a different scale, known as Brix (or Balling in Europe), which directly measures sugar content. While the concept is identical, the scale is different. Roughly speaking, 1 degree Brix is equivalent to .004 specific gravity points, so that a Brix of 0 equals 1.000 specific gravity, while a Brix of 24 degrees is equal to 1.100 specific gravity.
When a fermentation is started, the liquid will consist primarily of water, fruit solids (grape solids in the case of grape wine), and sugars. The specific gravity reading, which should be in the range of 1.075 to 1.090, will - with readily-available tables - tell you not only how much alcohol may be produced, but by measurement on a frequent basis, how the must is progressing to completion. By definition, water is the standard, and has a specific gravity of 1.000. That of pure ethyl alcohol is 0.792, so that a "dry" wine - one containing no sugar, just water, alcohol and some dissolved solids -will have a specific gravity of less than 1.000 (typically in the range of 0.990).
Over the course of the fermentation, readings drop, showing that sugar is being converted to alcohol. When the readings stabilize, and the total drop indicates that alcohol conversion is complete, the new wine is ready to be removed from the fruit and yeast debris it has thrown. A wine thief can be a helpful device for the home winemaker during fermentation, since it is wide enough to accommodate a hydrometer. It can be used to take samples as well as to take hydrometer readings while you ferment in a glass carboy. You can keep a thief-hydrometer in a carboy with sanitizer so you only need to rinse it off before using it.
A number of tables have been produced that attempt to relate initial sugar content to final alcohol level, but there is considerable disagreement between them. The reason for this is that some are based on a "laboratory situation" of a pure sucrose solution in water, whereas grape juice also includes "non-fermentable" substances that add to the specific gravity, but not to the alcohol content. Further, calculation of alcohol production depends on total drop in specific gravity during fermentation, not just on the starting specific gravity (a juice that ferments out to a final specific gravity of 1.010 will have a lower alcohol content than one which finishes at 0.992, if both started at the same specific gravity). Some tables take account of this by showing that a fermenting must at SG 1.000 actually still has some sugar present, while others do not take this into account and show no sugar content at this level. However, consistent use of the same table will allow you to achieve consistent results, which is more important than accuracy to three decimal places.
 An acid testing kit is the next most important item in your lab. Acids, as most people know, are chemicals which, in high concentrations, are caustic and damaging. Their chemical opposites are known as bases or alkalis (such as lye). These are also, paradoxically, caustic.
An acid testing kit is the next most important item in your lab. Acids, as most people know, are chemicals which, in high concentrations, are caustic and damaging. Their chemical opposites are known as bases or alkalis (such as lye). These are also, paradoxically, caustic.
When acids are dissolved in water, their molecules dissociate - break apart - with hydrogen ions being released into the liquid (some acids release more hydrogen ions than others, and thus are more "active" than others; food acids are typically very weak). Chemically, hydrogen ions have the symbol H+. Bases, on the other hand, dissociate by releasing hydroxyl ions, with the chemical symbol OH-. If the two encounter each other, they neutralize each other, combining to form plain water, while releasing energy in the form of heat.
Virtually all fruit contains acid, but fruit acids are mild, and are found in relatively small concentrations.In juices acid gives a sharpness or zest to the flavor; pineapple juice, for example, is higher in acid - and sharper in flavor - than the juice of ripe apples. In a wine, too much acid makes the taste unpleasantly sharp, but too little leaves it bland and uninteresting.
We can measure acid in two ways; in titratable acidity, which measures the total amount of fixed acid present, regardless of its strength; and in pH, which measures the activity or strength of an acid, regardless of its concentration. Although our taste is more correlated with acid activity than with its concentration, the latter is easier to measure, and - if we are dealing with the normal acids one experiences in food and drink - reasonably accurate.
Your home lab should therefore contain an acid titration kit. This consists of one or two Titrets - a syringe without needle, but calibrated in cubic centimetres (CCs), also known as milliliters (mL) - plus a plastic or glass testing jar and two containers of reagent (that's chem-speak for chemical solutions). One of these is sodium hydroxide, at a known strength of 1/5 normal (0.2N), and the other is phenolphthalein solution, which has the interesting property of being colorless in an acidic solution, but which turns red in an alkaline solution.
The process is simply to add a measured quantity of the sodium hydroxide to a measured quantity of the wine, which is acidic, and noting exactly when the color change occurs to indicate that all of the acid has been neutralized. This is a lot simpler than it seems and the process is as follows:
Using 15 mL of wine (to which 3 drops of phenolphthalein solution has been added) one adds, a small quantity at a time, a 0.2N sodium hydroxide solution (trust me; it works). The color change from white to pink indicates the neutralization of the acid. The magic formula is that the acid content of the wine, in grams per liter (or parts per thousand), exactly equals the number of mL of sodium hydroxide used; thus, 7.5 mL equals an acid content of 7.5 grams per liter, measured "as tartaric". Acid titration kits are available virtually wherever winemaking supplies and ingredients are sold.
Do be sure, though, that you are measuring apples with apples; British books use "as sulfuric" by their standards, and the values obtained are only 2/3 our measure of "as tartaric" equivalent. Also, as a rule of thumb, the sodium hydroxide should be replaced every six to nine months, but the phenolphthalein will last a couple of years.
 The strength of both acids and bases is measured by a yardstick known as pH, which is defined as the "common logarithm of the reciprocal of the hydrogen ion concentration" (don't worry about that, it won't be on the final exam; this mathematical legerdemain is required in order to yield a usable numeric range. Otherwise the number would have so many zeros as to be virtually incomprehensible). The value of pH ranges from 1 (highly caustic acid) to 14 (highly caustic base). Water - being neutral - lies in the middle with a pH of 7.0. The total amount of acid can also be measured as a percentage of the total volume of the must or wine, usually expressed in grams per liter (g/L), which is equal to parts per thousand, the measure used in industry. Total acid in this article means total titratable acid, or fixed acid.
The strength of both acids and bases is measured by a yardstick known as pH, which is defined as the "common logarithm of the reciprocal of the hydrogen ion concentration" (don't worry about that, it won't be on the final exam; this mathematical legerdemain is required in order to yield a usable numeric range. Otherwise the number would have so many zeros as to be virtually incomprehensible). The value of pH ranges from 1 (highly caustic acid) to 14 (highly caustic base). Water - being neutral - lies in the middle with a pH of 7.0. The total amount of acid can also be measured as a percentage of the total volume of the must or wine, usually expressed in grams per liter (g/L), which is equal to parts per thousand, the measure used in industry. Total acid in this article means total titratable acid, or fixed acid.
We measure pH using a pH meter. These meters come in various configurations, but unless one is prepared to pay top dollar for a laboratory model, check at a winemaking store or a scientific-supply firm for a vest-pocket styled model with digital readout. Typically, a red wine should show a pH of between 3.2 and 3.4, while a slightly lower value, representing a more acidic solution - between 3.0 and 3.2 - works well for whites.
You will need to calibrate a pH meter before each use; this is typically done by immersing the electrode in two solutions of known pH, one at pH 7.0 and the other at pH 4.0, and adjusting the reading on the meter until it reads accurately starting with the pH 7.0 standard. These solutions are made using distilled water and capsules of reagent, usually available from the same source as the meter. Since the standard solutions are not stable over time, they should be recreated each time the meter is put into use, unless the previous occasion was within several days. In addition, the adjusting mechanism on some meters is flimsy, for example, a countersunk screw made of soft metal that deforms after severaluses, so the meter can become impossible to calibrate.
All pH meters should be stored with the electrode kept damp, preferably in asolution made for this purpose by the manufacturer. A reasonably-priced pH meter should be accurate to one decimal place, for example, able to distinguish 3.2 from 3.3. It doesn't need to be as good as proper lab equipment, but it should be perfectly adequate for us amateurs.
For rough measurements of pH, litmus paper is an alternative. This is a chemically-treated paper strip which changes color when dipped in a wine, with the color being an indication of the pH. One simply dips the paper in the wine, waits for a few seconds, then compares the ensuing color with that on a chart, provided with the container. Matching up the color with one on the chart indicates the pH with a reasonable approximation - but not the same as a pH meter.
It should be emphasized that litmus paper only gives approximate results, depending as it does on both the quality of the product and your own ability to discriminate slight differences in color. However, as a fast means of determining changes in acidity during fermentation, and the direction in which the change is occurring, it is useful for a quick, rough-and-ready determination. For absolute accuracy, however, a pH meter is highly recommended when you are making the final determination.
Many of t he measurements you will make - such as specific gravity, but also starting conditions of your wine and ongoing ambient conditions during fermentation - are temperature-dependent. Two types of thermometers are advisable: the floating kind of thermometer, to measure temperatures in the must, and one other - which can be either a standard household one, or a liquid-crystal display - to indicate room conditions. Most supply stores sell thermometers, and if not, you can try a pet store or hardware store. Another option is strip thermometers, which can be stuck to a carboy or simply tacked to a shelf. They are readily available and give reasonably accurate results.
he measurements you will make - such as specific gravity, but also starting conditions of your wine and ongoing ambient conditions during fermentation - are temperature-dependent. Two types of thermometers are advisable: the floating kind of thermometer, to measure temperatures in the must, and one other - which can be either a standard household one, or a liquid-crystal display - to indicate room conditions. Most supply stores sell thermometers, and if not, you can try a pet store or hardware store. Another option is strip thermometers, which can be stuck to a carboy or simply tacked to a shelf. They are readily available and give reasonably accurate results.
 Balance scales are your next item of choice. My balance scale measures down to 1/10 gram. You can't get much finer than that. Why do you need one? Because in small quantities of wine production, which is what most home winemakers are involved in, it is sometimes necessary to measure additives in gram quantities, and an accurate scale is a great tool to have at these times - for example, when determining the quantity of oak chips to add (in an oaked white wine, typically 3 grams per liter). Kitchen scales work well for larger quantities, such as sugar addition, where gram accuracy is not really important.
Balance scales are your next item of choice. My balance scale measures down to 1/10 gram. You can't get much finer than that. Why do you need one? Because in small quantities of wine production, which is what most home winemakers are involved in, it is sometimes necessary to measure additives in gram quantities, and an accurate scale is a great tool to have at these times - for example, when determining the quantity of oak chips to add (in an oaked white wine, typically 3 grams per liter). Kitchen scales work well for larger quantities, such as sugar addition, where gram accuracy is not really important.
For volume measure, invest also in a measuring cup and, if available in your area, a graduated cylinder with liquid measure. There will be times when you want to add a few ounces of sugar and a measuring cup is more convenient than a scale. The graduated cylinder is handy when using recipes that require, for example, the setting aside of, say, 150 ml of "sweet reserve."
Ordinary measuring spoons will also make life easier. For conversion purposes, one teaspoon equals 5 ml (or CCs), and one tablespoon equals 15 ml/CCs. These are useful when one is adding small quantities of additives such as sulfite, tannin and yeast nutrient. You can do your own math for table and teaspoons.
SULFITE TITRETS
Ever wondered how much S02 you have in your wine? Try using, in your home lab, a set of sulfite Titrets. These consist of glass tubes containing a colorless liquid, to which you fit a thin plastic tube that is provided with the kit. The ampoule is then inserted into a plastic tool which snaps the thin glass wand at the end of the ampoule and then functions as a pump to draw a small quantity of the wine into the it. Initially the sample will turn blue, but after successive additions of the sample, the color changes back to colorless. The ampoules are marked with gradations from 10 to 100 miligrams per liter, and it is a simple matter to read the level from the engraved numbers. Titrets are available at home-winemaking stores or from CHEMetrics in Calverton, Virginia. (See "Solving the Sulfite Puzzle" in the Winter 2000 edition of WineMaker).
CHROMATOGRAPHY
Advanced and serious winemakers might consider chromatography. These identify the presence of even very small concentrations of a substance in a sample to be tested, by analyzing the characteristic (and unique) color "signature" they produce. Equally important, chromatography measures the approximate amount of the substance. The most accurate (and expensive) way of doing this is by gas chromatography, a method limited generally to large research laboratories or some university facilities. However, a simpler, and fairly easy to use, alternative is paper chromatography. A paper chromatography kit can be obtained from a scientific supply store, or one of the larger suppliers to home winemakers, with a current price reportedly under $40 US. Chromatography is a particularly useful science for determining the progress (and completion point) of malolactic fermentation, which is the technique of converting sharp-tasting malic acid into milder and more complex lactic acid through the use of a lactobacilli culture. It is frequently used in red wines and Chardonnays, which have a higher than desirable level of malic acid (which has a taste reminiscent of unripe apples, and mars the flavor profile of reds and Chards). The chromatography kit can be used to determine when the malic acid has been totally converted to lactic, since the malic "signature" will disappear and the lactic "signature" will appear instead.
The technique for using the chromatograph involves putting a small drop of wine on a blotter and then standing it up in about an inch of developer liquid, preferably in a large jar with a lid. After a duration of six hours, the blotter is taken out and developed. The results are determined by using the instructions that come with the kit. The home winemaker can probably test over 100 wines with this method before needing more developer solution, which costs about $10 US.
KEEP IT CLEAN
Sterilants are a primary ingredient of your lab. A fermenting wine must provides an environment particularly suited to the growth of fungi and bacteria which, if unchecked, can spoil the wine. Dirty or contaminated equipment, or surroundings, can infect the wine either directly (in the case of equipment) or indirectly by providing a medium in which spoilage organisms can proliferate.
Sterilants come in a variety of types, each of which is specific to a particular function. Some include a detergent to assist in removing foreign matter. Examples of these are the commercial products Sanitone and Chloriclean. (Chloriclean is widely available in winemaker supply stores, but you might also want to check with your local dairy supplier for a bulk purchase; farmer co-ops also often have sterilants that those of us in the suburbs don't know about.) When used on equipment or containers that will come in contact with the wine, these must first be thoroughly rinsed away so that no noticeable trace of the detergent remains.
Chlorine-based sterilants, such as Clorox and similar products, sterilize by bleaching the offending matter or stain. This process of denaturing destroys the integrity of the material, which can then be readily rinsed away. Chlorine bleaches are particularly useful for removing stains from bottles or carboys, or cleaning up after a spill in the winery. However, they can cause irritation of the nasal passages or eyes in a confined space and, when used on equipment with which the wine will come into contact, must also be thoroughly rinsed away.
Note: Chlorine bleaches should not be used on stainless-steel items because they will corrode the metal. Second-hand barrels, which are generally not a good idea (see "Wooden it be Lovely" in the Fall 2000 issue of WineMaker) require different treatment if you are determined to use them. Older books suggest a combination of soda ash and household lye; others suggest washing soda at the rate of 1 pound per 3 gallons of hot water, as a means of removing scale from inside the barrel. Again it is crucial that these should be thoroughly rinsed out before using the cask.
Sulfur-based sterilants are used to destroy bacteria and fungi only, and do not convey any significant cleansing action. The old technique for keeping a clean barrel "sweet" was to burn a sulfur stick below the bung-hole of a barrel placed upside-down on its cradle; alternatively, a metal "spoon" fixed to a long handle, in an L-shaped form, was filled with powdered sulfur, set alight, and lowered into the barrel. This released a strong concentration of S02 fumes which destroyed any bacteria present.
The current sanitation method of choice for most winemaking equipment, including clean barrels, is a concentrated solution of potassium (or sodium) metabisulfite. I use one teaspoon in six U.S. gallons of water, which gives a concentration of 100 parts per million (ppm) of SO2. Other winemakers may prefer higher concentrations. When using higher concentrations, you might want to rinse your equipment with water before using it. If you use a lower concentration - say, a half-teaspoon of metabisulfite in six gallons of water, which equates to about 50 ppm - you can simply drain the equipment. The small amount that may be left after draining a sanitized wine bottle, for example, is unnoticeable and provides a measure of protection while the bottle is being filled.
RECORD KEEPING
Keeping a record of your results is an important, yet often overlooked, aspect of winemaking. In addition to making a list of ingredients - including source and cost of each ingredient - it's a good idea to note the amount of acid, tannin and nutrient added, and the yeast variety used. You should also record the starting specific gravity and acid readings, the date fermentation began, any observations as fermentation progresses, dates of each racking, any additions during fermentation (such as sugar, sulfite, stabilizer and sweet reserve), fining agents or filtering, and any temperature corrections.
Depending on your expectations or level of motivation, your records may be as elaborate as a computer database or a commercially-produced log book, or as simple as a card index. (Also see the downloadable log chart here at the website.) Having tried all three versions, I prefer the latter. A book of tasting results, which can be used both for your own wine and commercially-produced wine, will also bring back fond memories of the superior wines you have encountered, and, of course, enhance your bragging rights.
THE NOSE KNOWS
The most important instrument you can employ is your nose. Sadly, we often fail to use the best instrument we possess ... our own senses!


